Cardiac Autonomic Dysfunction in Multiple Sclerosis: A Systematic Review of Current Knowledge and Impact of Immunotherapies
Abstract
:1. Introduction
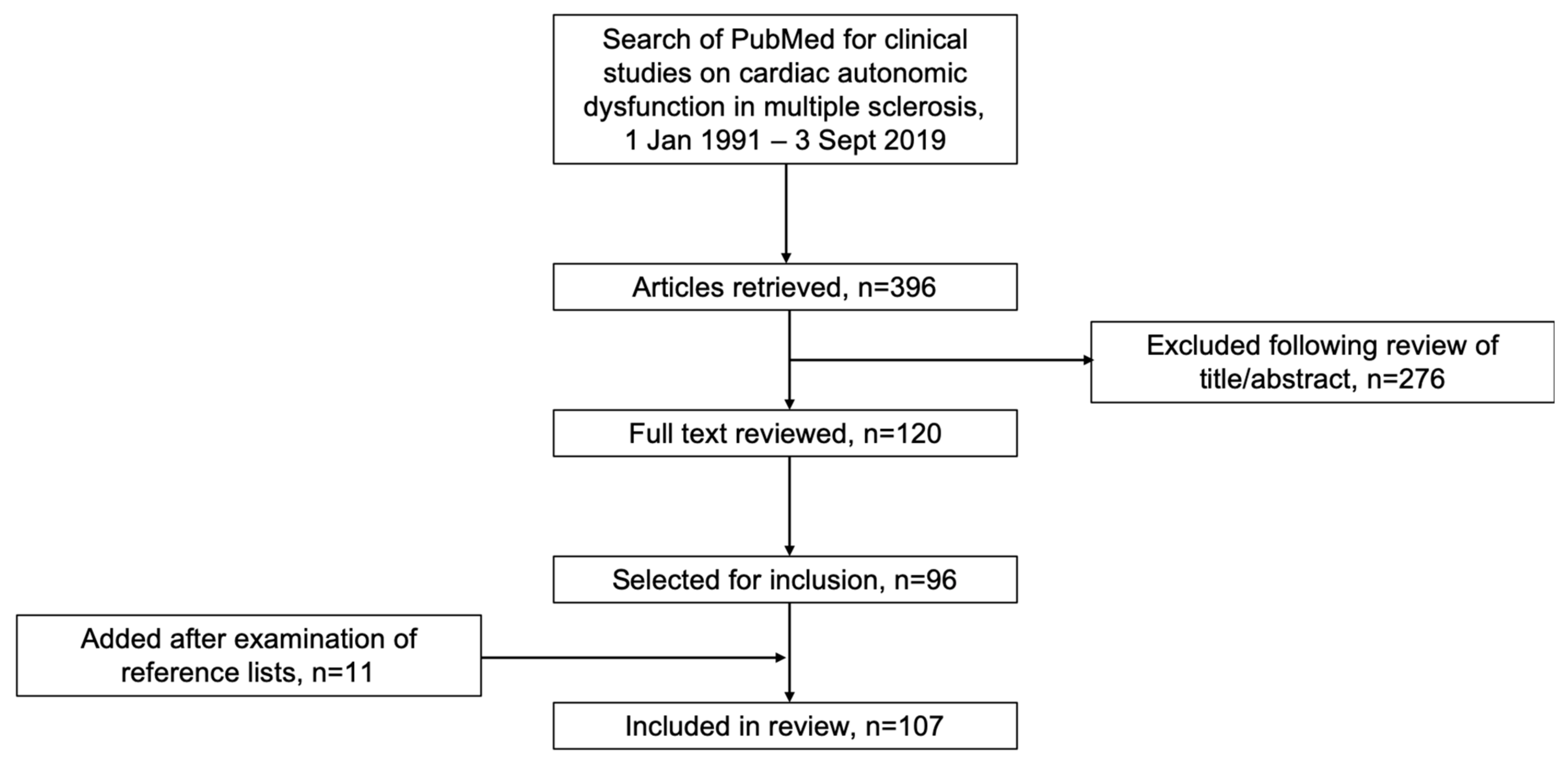
2. Literature Search
3. CAD in MS
3.1. Testing for CAD in MS
3.2. Prevalence, Findings and Types of CAD in MS
3.3. Symptoms of CAD in MS
3.4. Association with MS Type and Disease Characteristics
3.5. Association with Fatigue and Cognitive Impairment
3.6. Treatment of CAD in MS
3.7. Possible Mechanisms of CAD in MS
3.8. Association with MRI Lesions
3.8.1. Brainstem/Infratentorial Lesions
3.8.2. Supratentorial Lesions
3.8.3. Spinal Cord Lesions
4. CAD Associated with Immunotherapies in MS
4.1. Sphingosine 1-Phosphate (S1P) Receptor Modulators
4.1.1. Fingolimod
4.1.2. Siponimod
4.1.3. Ozanimod
4.1.4. Mechanism
4.2. Other MS Drugs
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Racosta, J.M.; Kimpinski, K.; Morrow, S.A.; Kremenchutzky, M. Autonomic dysfunction in multiple sclerosis. Auton. Neurosci. 2015, 193, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Sanchez, C.; Goodman, B.P.; Carter, J.L.; Wingerchuk, D.M. The spectrum of acute cardiopulmonary events associated with multiple sclerosis exacerbations. Mult. Scler. J. 2019, 25, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Racosta, J.M.; Kimpinski, K. Autonomic dysfunction, immune regulation, and multiple sclerosis. Clin. Auton. Res. 2016, 26, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Shirbani, F.; Barin, E.; Lee, Y.-C.; Ng, K.; Parratt, J.D.E.; Butlin, M.; Avolio, A.P. Characterisation of cardiac autonomic function in multiple sclerosis based on spontaneous changes of heart rate and blood pressure. Mult. Scler. Relat. Disord. 2018, 22, 120–127. [Google Scholar] [CrossRef]
- Flachenecker, P.; Reiners, K.; Krauser, M.; Wolf, A.; Toyka, K.V. Autonomic dysfunction in multiple sclerosis is related to disease activity and progression of disability. Mult. Scler. J. 2001, 7, 327–334. [Google Scholar] [CrossRef]
- Habek, M. Immune and autonomic nervous system interactions in multiple sclerosis: Clinical implications. Clin. Auton. Res. 2019, 29, 267–275. [Google Scholar] [CrossRef]
- Racosta, J.M.; Sposato, L.A.; Morrow, S.A.; Cipriano, L.E.; Kimpiski, K.; Kremenchutzky, M. Cardiovascular autonomic dysfunction in multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2015, 4, 104–111. [Google Scholar] [CrossRef]
- Merkelbach, S.; Dillmann, U.; Kölmel, C.; Holz, J.; Müller, M. Cardiovascular autonomic dysregulation and fatigue in multiple sclerosis. Mult. Scler. J. 2001, 7, 320–326. [Google Scholar] [CrossRef]
- Christiansen, C.F.; Christensen, S.; Farkas, D.K.; Miret, M.; Sørensen, H.T.; Pedersen, L. Risk of Arterial Cardiovascular Diseases in Patients with Multiple Sclerosis: A Population-Based Cohort Study. Neuroepidemiology 2010, 35, 267–274. [Google Scholar] [CrossRef]
- Kaplan, T.B.; Berkowitz, A.L.; Samuels, M.A. Cardiovascular Dysfunction in Multiple Sclerosis. The Neurologist 2015, 20, 108–114. [Google Scholar] [CrossRef]
- Scalfari, A.; Knappertz, V.; Cutter, G.; Goodin, D.S.; Ashton, R.; Ebers, G.C. Mortality in patients with multiple sclerosis. Neurology 2013, 81, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Hilz, M.J. Cardiac stunning as first manifestation of multiple sclerosis: A case report reminding us not to overlook cardiovascular autonomic dysfunction in multiple sclerosis. Mult. Scler. J. 2016, 22, 847–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midaglia, L.; Juega Mariño, J.M.; Sastre-Garriga, J.; Rovira, A.; Vidal-Jordana, A.; López-Pérez, M.A.; Montalban, X. An uncommon first manifestation of multiple sclerosis: Tako-Tsubo cardiomyopathy. Mult. Scler. J. 2016, 22, 842–846. [Google Scholar] [CrossRef]
- London, F.; Gonzalez Rodriguez de Azero, N.; Philippart, M.; Higny, J.; Mulquin, N. Reverse takotsubo cardiomyopathy triggered by a multiple sclerosis relapse. Acta Neurol. Belg. 2019, 119, 295–297. [Google Scholar] [CrossRef]
- Madias, J.E. Takotsubo syndrome triggered in the setting of multiple sclerosis: The need to monitor blood catecholamines and the CNS sympathetic input to the heart. Mult. Scler. Relat. Disord. 2019, 27, 391. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.D.; Cahill, J.F.; Rizvi, S.A. Multiple sclerosis relapse presenting as an acute cardiomyopathy. Mult. Scler. Relat. Disord. 2019, 27, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Habek, M.; Crnošija, L.; Gabelić, T.; Barun, B.; Adamec, I.; Junaković, A.; Ruška, B.; Pavičić, T.; Skorić, M.K. Longitudinal assessment of autonomic nervous system in patients with first demyelinating event suggestive of multiple sclerosis. Eur. J. Neurol. 2019, 26, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Skorić, M.K.; Crnošija, L.; Gabelić, T.; Barun, B.; Adamec, I.; Junaković, A.; Pavičić, T.; Ruška, B.; Habek, M. Autonomic symptom burden can predict disease activity in early multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 28, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Rosini, J.M.; Rajasimhan, S.; Fellows, S.E.; Nomura, J.T. Delayed cardiac dysrhythmias after fingolimod administration. Am. J. Emerg. Med. 2015, 33, 987.e1. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, P.S.; Berger, J.R. Delayed fingolimod-associated asystole. Mult. Scler. J. 2011, 17, 1387–1389. [Google Scholar] [CrossRef]
- Lindsey, J.; Haden-Pinneri, K.; Memon, N.; Buja, L.M. Sudden unexpected death on fingolimod. Mult. Scler. J. 2012, 18, 1507–1508. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.; Schernthaner, C.; Wipfler, P.; Sellner, J. Delayed high-grade atrioventricular block requiring pacemaker implantation in a multiple sclerosis patient treated with fingolimod. Mult. Scler. Relat. Disord. 2020, 38, 101515. [Google Scholar] [CrossRef] [PubMed]
- Koçer, A.; Karakaya, O.; Kargin, R.; Barutcu, I.; Esen, A.M. P wave duration and dispersion in multiple sclerosis. Clin. Auton. Res. 2005, 15, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Razazian, N.; Hedayati, N.; Moradian, N.; Bostani, A.; Afshari, D.; Asgari, N. P wave duration and dispersion and QT interval in multiple sclerosis. Mult. Scler. Relat. Disord. 2014, 3, 662–665. [Google Scholar] [CrossRef] [Green Version]
- Turri, G.; Calabrese, M.; Pancheri, E.; Monaco, S.; Gajofatto, A.; Marafioti, V. QTc interval in patients with multiple sclerosis: An inference from the insula of Reil? Eur. J. Neurol. 2017, 24, 491–496. [Google Scholar] [CrossRef]
- Kodounis, A.; Stamboulis, E.; Constantinidis, T.S.; Liolios, A. Measurement of autonomic dysregulation in multiple sclerosis. Acta Neurol. Scand. 2005, 112, 403–408. [Google Scholar] [CrossRef]
- Hale, L.A.; Nukada, H.; Du Plessis, L.J.; Peebles, K.C. Clinical screening of autonomic dysfunction in multiple sclerosis. Physiother. Res. Int. 2009, 14, 42–55. [Google Scholar] [CrossRef]
- Videira, G.; Castro, P.; Vieira, B.; Filipe, J.P.; Santos, R.; Azevedo, E.; Sá, M.J.; Abreu, P. Autonomic dysfunction in multiple sclerosis is better detected by heart rate variability and is not correlated with central autonomic network damage. J. Neurol. Sci. 2016, 367, 133–137. [Google Scholar] [CrossRef]
- Diamond, B.; Kim, H.; DeLuca, J.; Cordero, D. Cardiovascular regulation in multiple sclerosis. Mult. Scler. J. 1995, 1, 156–162. [Google Scholar] [CrossRef]
- Frontoni, M.; Fiorini, M.; Strano, S.; Cerutti, S.; Giubilei, F.; Urani, C.; Bastianello, S.; Pozzilli, C. Power spectrum analysis contribution to the detection of cardiovascular dysautonomia in multiple sclerosis. Acta Neurol. Scand. 1996, 93, 241–245. [Google Scholar] [CrossRef]
- Linden, D.; Diehl, R.R.; Kretzschmar, A.; Berlit, P. Autonomic evaluation by means of standard tests and power spectral analysis in multiple sclerosis. Muscle Nerve 1997, 20, 809–814. [Google Scholar] [CrossRef]
- Brezinova, M.; Goldenberg, Z.; Kucera, P. Autonomic nervous system dysfunction in multiple sclerosis patients. Bratisl. Lek. Listy 2004, 105, 404–407. [Google Scholar] [PubMed]
- Ferini-Strambi, L.; Rovaris, M.; Oldani, A.; Martinelli, V.; Filippi, M.; Smirne, S.; Zucconi, M.; Comi, G. Cardiac autonomic function during sleep and wakefulness in multiple sclerosis. J. Neurol. 1995, 242, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.M.; Fadel, P.J.; Harnsberger, M.A.; Remington, G.M.; Frohman, E.M.; Davis, S.L. Reduced spontaneous sympathetic nerve activity in multiple sclerosis patients. J. Neurol. Sci. 2014, 344, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Monge, A.J.A.; Palacios, O.F.; Vila-Sobrino, J.A.; Matias-Guiu, J. Heart rate variability in multiple sclerosis during a stable phase. Acta Neurol. Scand. 1998, 97, 86–92. [Google Scholar] [CrossRef]
- Giubilei, F.; Vitale, A.; Urani, C.; Fronton, M.; Fiorini, M.; Millefiorini, E.; Fiorelli, M.; Santini, M.; Strano, S.; Frontoni, M. Cardiac Autonomic Dysfunction in Relapsing-Remitting Multiple Sclerosis during a Stable Phase. Eur. Neurol. 1996, 36, 211–214. [Google Scholar] [CrossRef]
- Tombul, T.; Anlar, O.; Tuncer, M.; Huseyinoglu, N.; Eryonucu, B. Impaired heart rate variability as a marker of cardiovascular autonomic dysfunction in multiple sclerosis. Acta Neurol. Belg. 2011, 111, 116–120. [Google Scholar]
- Ganz, R.E.; Weibels, G.; Stäcker, K.-H.; Faustmann, P.M.; Zimmermann, C.W. The Lyapunov Exponent of Heart Rate Dynamics as a Sensitive Marker of Central Autonomic Organization: An Exemplary Study of Early Multiple Sclerosis. Int. J. Neurosci. 1993, 71, 29–36. [Google Scholar] [CrossRef]
- Habek, M.; Crnošija, L.; Lovrić, M.; Junaković, A.; Skorić, M.K.; Adamec, I. Sympathetic cardiovascular and sudomotor functions are frequently affected in early multiple sclerosis. Clin. Auton. Res. 2016, 26, 385–393. [Google Scholar] [CrossRef]
- Al-Araji, A.H.; Al-Mahdawi, A.M.; Mohammad, A.I. Autonomic dysfunction in multiple sclerosis. Neurosciences 2003, 8, 177–183. [Google Scholar]
- Gunal, D.I.; Afsar, N.; Tanridag, T.; Aktan, S. Autonomic dysfunction in multiple sclerosis: Correlation with disease-related parameters. Eur. Neurol. 2002, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Thomaides, T.N.; Zoukos, Y.; Chaudhuri, K.R.; Mathias, C.J. Physiological assessment of aspects of autonomic function in patients with secondary progressive multiple sclerosis. J. Neurol. 1993, 240, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Saari, A.; Tolonen, U.; Pääkkö, E.; Suominen, K.; Pyhtinen, J.; Sotaniemi, K.; Myllyla, V. Cardiovascular autonomic dysfunction correlates with brain MRI lesion load in MS. Clin. Neurophysiol. 2004, 115, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Gervasoni, E.; Bove, M.; Sinatra, M.; Grosso, C.; Rovaris, M.; Cattaneo, D.; Merati, G. Cardiac autonomic function during postural changes and exercise in people with multiple sclerosis: A cross-sectional study. Mult. Scler. Relat. Disord. 2018, 24, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Adamec, I.; Crnošija, L.; Junaković, A.; Skorić, M.K.; Habek, M. Progressive multiple sclerosis patients have a higher burden of autonomic dysfunction compared to relapsing remitting phenotype. Clin. Neurophysiol. 2018, 129, 1588–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Seze, J.; Stojkovic, T.; Gauvrit, J.-Y.; Devos, D.; Ayachi, M.; Cassim, F.; Michel, T.S.; Pruvo, J.-P.; Guieu, J.-D.; Vermersch, P. Autonomic dysfunction in multiple sclerosis: Cervical spinal cord atrophy correlates. J. Neurol. 2001, 248, 297–303. [Google Scholar] [CrossRef]
- Vita, G.; Fazio, M.C.; Milone, S.; Blandino, A.; Salvi, L.; Messina, C. Cardiovascular autonomic dysfunction in multiple sclerosis is likely related to brainstem lesions. J. Neurol. Sci. 1993, 120, 82–86. [Google Scholar] [CrossRef]
- Brinar, V.; Brzović, Z.; Papa, J.; Malojcić, B.; Dawidowsky, K. Autonomic dysfunction in patients with multiple sclerosis. Coll. Antropol. 1997, 21, 493–497. [Google Scholar]
- Mahovic, D.; Lakusic, N. Progressive Impairment of Autonomic Control of Heart Rate in Patients with Multiple Sclerosis. Arch. Med. Res. 2007, 38, 322–325. [Google Scholar] [CrossRef]
- Damla, O.; Altug, C.; Pinar, K.K.; Alper, K.; Dilek, I.G.; Kadriye, A. Heart rate variability analysis in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 24, 64–68. [Google Scholar] [CrossRef]
- Vlcek, M.; Penesova, A.; Imrich, R.; Meskova, M.; Mravcova, M.; Grunnerova, L.; Garafova, A.; Sivakova, M.; Turcani, P.; Kollar, B.; et al. Autonomic Nervous System Response to Stressors in Newly Diagnosed Patients with Multiple Sclerosis. Cell. Mol. Neurobiol. 2018, 38, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, K.; TenVoorde, B.J.; Adèr, H.J.; Uitdehaag, B.; Polman, C.H. Longitudinal follow-up of cardiovascular reflex tests in multiple sclerosis. J. Neurol. Sci. 1998, 155, 50–54. [Google Scholar] [CrossRef]
- Nasseri, K.; Uitdehaag, B.M.J.; Walderveen, M.A.; Adèr, H.J.; Polman, C.H. Cardiovascular autonomic function in patients with relapsing remitting multiple sclerosis: A new surrogate marker of disease evolution? Eur. J. Neurol. 1999, 6, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Krbot, S.M.; Crnosija, L.; Adamec, I.; Barun, B.; Gabelic, T.; Smoljo, T.; Stanic, I.; Pavicic, T.; Pavlovic, I.; Drulovic, J.; et al. Autonomic symptom burden is an independent contributor to multiple sclerosis related fatigue. Clin. Auton. Res. 2019, 29, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Lebre, A.T.; Mendes, M.F.; Tilbery, C.P.; Almeida, A.L.; Neto, A.S. Relation between fatigue and autonomic disturbances in multiple sclerosis. Arq. Neuro Psiquiatr. 2007, 65, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Flachenecker, P.; Rufer, A.; Bihler, I.; Hippel, C.; Reiners, K.; Toyka, K.; Kesselring, J. Fatigue in MS is related to sympathetic vasomotor dysfunction. Neurology 2003, 61, 851–853. [Google Scholar] [CrossRef]
- Keselbrener, L.; Akselrod, S.; Ahiron, A.; Eldar, M.; Barak, Y.; Rotstein, Z. Is fatigue in patients with multiple sclerosis related to autonomic dysfunction? Clin. Auton. Res. 2000, 10, 169–175. [Google Scholar] [CrossRef]
- Heesen, C.; Koehler, G.; Gross, R.; Tessmer, W.; Schulz, K.-H.; Gold, S.M. Altered cytokine responses to cognitive stress in multiple sclerosis patients with fatigue. Mult. Scler. J. 2005, 11, 51–57. [Google Scholar] [CrossRef]
- Sander, C.; Modes, F.; Schlake, H.-P.; Eling, P.; Hildebrandt, H. Capturing fatigue parameters: The impact of vagal processing in multiple sclerosis related cognitive fatigue. Mult. Scler. Relat. Disord. 2019, 32, 13–18. [Google Scholar] [CrossRef]
- Niepel, G.; Bibani, R.H.; Vilisaar, J.; Langley, R.W.; Bradshaw, C.M.; Szabadi, E.; Constantinescu, C.S. Association of a deficit of arousal with fatigue in multiple sclerosis: Effect of modafinil. Neuropharmacology 2013, 64, 380–388. [Google Scholar] [CrossRef]
- Arata, M.; Sternberg, Z. Transvascular Autonomic Modulation: A Modified Balloon Angioplasty Technique for the Treatment of Autonomic Dysfunction in Multiple Sclerosis Patients. J. Endovasc. Ther. 2014, 21, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Allen, D.R.; Keller, D.M.; Fadel, P.J.; Frohman, E.M.; Davis, S.L. Impaired carotid baroreflex control of arterial blood pressure in multiple sclerosis. J. Neurophysiol. 2016, 116, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanya, E.O.; Tutaj, M.; Brown, C.M.; Goel, N.; Neundörfer, B.; Hilz, M.J.; Sanya, E.O. Abnormal heart rate and blood pressure responses to baroreflex stimulation in multiple sclerosis patients. Clin. Auton. Res. 2005, 15, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Winder, K.; Linker, R.A.; Seifert, F.; Wang, R.; Lee, D.; Engelhorn, T.; Dörfler, A.; Fröhlich, K.; Hilz, M. Cerebral lesion correlates of sympathetic cardiovascular activation in multiple sclerosis. Hum. Brain Mapp. 2019, 40, 5083–5093. [Google Scholar] [CrossRef] [Green Version]
- Simula, S.; Laitinen, T.; Laitinen, T.M.; Tarkiainen, T.; Hartikainen, P.; Hartikainen, J.E. Effect of fingolimod on cardiac autonomic regulation in patients with multiple sclerosis. Mult. Scler. J. 2016, 22, 1080–1085. [Google Scholar] [CrossRef]
- Vehoff, J.; Haegele-Link, S.; Humm, A.; Kaegi, G.; Mueller, S.K.; Sauter, R.; Tettenborn, B.E.; Hundsberger, T. Heart rate variability decreases after 3 months of sustained treatment with fingolimod. J. Neurol. 2017, 264, 2313–2317. [Google Scholar] [CrossRef]
- Akbulak, R.Ö.; Rosenkranz, S.C.; Schaeffer, B.N.; Pinnschmidt, H.O.; Willems, S.; Heesen, C.; Hoffmann, B.A. Acute and long-term effects of fingolimod on heart rhythm and heart rate variability in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 19, 44–49. [Google Scholar] [CrossRef]
- Rossi, S.; Rocchi, C.; Studer, V.; Motta, C.; Lauretti, B.; Germani, G.; Macchiarulo, G.; Marfia, G.A.; Centonze, D. The autonomic balance predicts cardiac responses after the first dose of fingolimod. Mult. Scler. J. 2015, 21, 206–216. [Google Scholar] [CrossRef]
- Li, K.; Konofalska, U.; Akgün, K.; Reimann, M.; Rüdiger, H.; Haase, R.; Ziemssen, T. Modulation of Cardiac Autonomic Function by Fingolimod Initiation and Predictors for Fingolimod Induced Bradycardia in Patients with Multiple Sclerosis. Front. Mol. Neurosci. 2017, 11, 540. [Google Scholar] [CrossRef]
- Vanoli, E.; Montano, N.; De Angelis, G.; Badilini, F.; Mirabella, M.; Bonavita, S.; Patti, F.; Bianco, A.; Sparaco, M.; Chisari, C.; et al. Cardiovascular autonomic individual profile of relapsing-remitting multiple sclerosis patients and risk of extending cardiac monitoring after first dose fingolimod. J. Neurol. Sci. 2019, 405, 116423. [Google Scholar] [CrossRef]
- Hilz, M.J.; Intravooth, T.; Moeller, S.; Wang, R.; Lee, D.-H.; Koehn, J.; Linker, R.A. Central Autonomic Dysfunction Delays Recovery of Fingolimod Induced Heart Rate Slowing. PLoS ONE 2015, 10, e0132139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, J.Q.; Hartung, J.P.; Olson, A.D.; Mendzelevski, B.; Timony, G.A.; Boehm, M.F.; Peach, R.J.; Gujrathi, S.; Frohna, P.A. Cardiac Safety of Ozanimod, a Novel Sphingosine-1-Phosphate Receptor Modulator: Results of a Thorough QT/QTc Study. Clin. Pharmacol. Drug Dev. 2018, 7, 263–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racca, V.; Rovaris, M.; Cavarretta, R.; Vaini, E.; Toccafondi, A.; Di Rienzo, M. Acute Fingolimod Effects on Baroreflex and Cardiovascular Autonomic Control in Multiple Sclerosis. J. Cent. Nerv. Syst. Dis. 2019, 11, 1179573519849945. [Google Scholar] [CrossRef] [PubMed]
- Hilz, M.J.; Wang, R.; Leal, C.D.R.; Liu, M.; Canavese, F.; Roy, S.; Hösl, K.M.; Winder, K.; Lee, D.-H.; Linker, R.A. Fingolimod initiation in multiple sclerosis patients is associated with potential beneficial cardiovascular autonomic effects. Ther. Adv. Neurol. Disord. 2017, 10, 191–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olindo, S.; Guillon, B.; Helias, J.; Phillibert, B.; Magne, C.; Feve, J.R. Decrease in heart ventricular ejection fraction during multiple sclerosis. Eur. J. Neurol. 2002, 9, 287–291. [Google Scholar] [CrossRef]
- Kale, N.; Magana, S.; Agaoglu, J.; Tanik, O.; Minelli, C.; Gondim, F.A.; Barreira, A.A.; Dromerick, A.W. Assessment of autonomic nervous system dysfunction in multiple sclerosis and the association with clinical disability. Neurol. Int. 2009, 1, 4. [Google Scholar] [CrossRef]
- Flachenecker, P.; Wolf, A.; Krauser, M.; Hartung, H.-P.; Reiners, K. Cardiovascular autonomic dysfunction in multiple sclerosis: Correlation with orthostatic intolerance. J. Neurol. 1999, 246, 578–586. [Google Scholar] [CrossRef]
- Kanjwal, K. Autonomic Dysfunction Presenting as Postural Orthostatic Tachycardia Syndrome in Patients with Multiple Sclerosis. Int. J. Med. Sci. 2010, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Saint-Croix, G.; Colombo, R. Brugada Syndrome Caused by Autonomic Dysfunction in Multiple Sclerosis. Case Rep. Cardiol. 2019, 2019. [Google Scholar] [CrossRef]
- Jurić, S.; Mišmaš, A.; Mihić, N.; Barać, A.M.; Habek, M. Newly onset sinus bradycardia in the context of multiple sclerosis relapse. Intern. Med. 2012, 51, 1121–1124. [Google Scholar] [CrossRef] [Green Version]
- Studer, V.; Rocchi, C.; Motta, C.; Lauretti, B.; Perugini, J.; Brambilla, L.; Centonze, D. Heart rate variability is differentially altered in multiple sclerosis: Implications for acute, worsening and progressive disability. Mult. Scler. J. Exp. Transl. Clin. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Ziqber, J.; Chmielewski, H.; Dryjariski, T.; Goch, J.H. Evaluation of myocardial muscle functional parameters in patients with multiple sclerosis. Acta Neurol. Scand. 1997, 95, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, A.; Ojanen, A.; Hartikainen, J.E.K.; Remes, A.M.; Simula, S. The impact of multiple sclerosis onset symptom on cardiac repolarization. Brain Behav. 2017, 7, e00742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkola, A.; Ojanen, A.; Hartikainen, J.E.; Remes, A.M.; Simula, S. Cardiac repolarization evolves differently during the course of benign and disabling multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 20, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, R.; Mori, M.; Fukutake, T.; Kita, K.; Hattori, T. Orthostatic hypotension in a case with multiple sclerosis. Clin. Auton. Res. 1997, 7, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Schroth, W.S.; Tenner, S.M.; Rappaport, B.A.; Mani, R. Multiple Sclerosis as a Cause of Atrial Fibrillation and Electrocardiographic Changes. Arch. Neurol. 1992, 49, 422–424. [Google Scholar] [CrossRef]
- Kappos, L.; Antel, J.; Comi, G.; Montalban, X.; O’Connor, P.; Polman, C.H.; Haas, T.; Korn, A.A.; Karlsson, G.; Radue, E.W. Oral Fingolimod (FTY720) for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2006, 355, 1124–1140. [Google Scholar] [CrossRef] [Green Version]
- Kappos, L.; Radue, E.-W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A Placebo-Controlled Trial of Oral Fingolimod in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.A.; Barkhof, F.; Comi, G.; Hartung, H.-P.; Khatri, B.O.; Montalban, X.; Pelletier, J.; Capra, R.; Gallo, P.; Izquierdo, G.; et al. Oral Fingolimod or Intramuscular Interferon for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2010, 362, 402–415. [Google Scholar] [CrossRef]
- Kappos, L.; O’Connor, P.; Radue, E.-W.; Polman, C.; Hohlfeld, R.; Selmaj, K.; Ritter, S.; Schlosshauer, R.; Von Rosenstiel, P.; Zhang-Auberson, L.; et al. Long-term effects of fingolimod in multiple sclerosis: The randomized FREEDOMS extension trial. Neurology 2015, 84, 1582–1591. [Google Scholar] [CrossRef] [Green Version]
- Calabresi, P.A.; Radue, E.-W.; Goodin, U.; Jeffery, U.; Rammohan, K.W.; Reder, A.T.; Vollmer, T.; Agius, M.A.; Kappos, L.; Stites, T.; et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014, 13, 545–556. [Google Scholar] [CrossRef]
- Kappos, L.; Cohen, J.; Collins, W.; De Vera, A.; Zhang-Auberson, L.; Ritter, S.; Von Rosenstiel, P.; Francis, G. Fingolimod in relapsing multiple sclerosis: An integrated analysis of safety findings. Mult. Scler. Relat. Disord. 2014, 3, 494–504. [Google Scholar] [CrossRef]
- DiMarco, J.P.; O’Connor, P.; Cohen, J.A.; Reder, A.T.; Zhang-Auberson, L.; Tang, D.; Collins, W.; Kappos, L. First-dose effects of fingolimod: Pooled safety data from three phase 3 studies. Mult. Scler. Relat. Disord. 2014, 3, 629–638. [Google Scholar] [CrossRef]
- Gold, R.; Comi, G.; Palace, J.; Siever, A.; Gottschalk, R.; Bijarnia, M.; von Rosenstiel, P.; Tomic, D.; Kappos, L. Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: A phase 3b, open-label study. J. Neurol. 2014, 261, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Laroni, A.; Brogi, D.; Morra, V.B.; Guidi, L.; Pozzilli, C.; Comi, G.; Lugaresi, A.; Turrini, R.; Raimondi, D.; Uccelli, A.; et al. Safety of the first dose of fingolimod for multiple sclerosis: Results of an open-label clinical trial. BMC Neurol. 2014, 14, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limmroth, V.; Ziemssen, T.; Lang, M.; Richter, S.; Wagner, B.; Haas, J.; Schmidt, S.; Gerbershagen, K.; Lassek, C.; Klotz, L.; et al. Electrocardiographic assessments and cardiac events after fingolimod first dose—A comprehensive monitoring study. BMC Neurol. 2017, 17, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ontaneda, D.; Hara-Cleaver, C.; Rudick, R.A.; Cohen, J.A.; Bermel, R.A. Early tolerability and safety of fingolimod in clinical practice. J. Neurol. Sci. 2012, 323, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Fragoso, Y.D.; Arruda, C.C.; Arruda, W.O.; Brooks, J.B.B.; Damasceno, A.; Damasceno, C.A.D.A.; Finkelsztejn, A.; Finkelsztejn, J.; Da Gama, P.D.; Giacomo, M.C.B.; et al. The real-life experience with cardiovascular complications in the first dose of fingolimod for multiple sclerosis. Arq. Neuro Psiquiatr. 2014, 72, 712–714. [Google Scholar] [CrossRef] [Green Version]
- Paolicelli, D.; Manni, A.; DiRenzo, V.; D’Onghia, M.; Tortorella, C.; Zoccolella, S.; Trojano, M. Long term cardiac safety and tolerability of Fingolimod in Multiple Sclerosis: A post-marketing study. J. Clin. Pharmacol. 2015, 55, 1131–1136. [Google Scholar] [CrossRef]
- Castillo-Trivino, T.; Lopetegui, I.; De Munain, A.L.; Olascoaga, J.; Alarcón-Duque, J.A. Ventricular tachycardia on chronic fingolimod treatment for multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 931–932. [Google Scholar] [CrossRef]
- Kocyigit, D.; Yalcin, M.U.; Gurses, K.M.; Tokgozoglu, L.; Karabudak, R. Are there any clinical and electrocardiographic predictors of heart rate reduction in relapsing- remitting multiple sclerosis patients treated with fingolimod? Mult. Scler. Relat. Disord. 2019, 27, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Selmaj, K.; Li, D.K.; Hartung, H.-P.; Hemmer, B.; Kappos, L.; Freedman, M.S.; Stuve, O.; Rieckmann, P.; Montalban, X.; Ziemssen, T.; et al. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): An adaptive, dose-ranging, randomised, phase 2 study. Lancet Neurol. 2013, 12, 756–767. [Google Scholar] [CrossRef]
- Kappos, L.; Li, D.K.B.; Stüve, O.; Hartung, H.-P.; Freedman, M.S.; Hemmer, B.; Rieckmann, P.; Montalban, X.; Ziemssen, T.; Hunter, B.; et al. Safety and Efficacy of Siponimod (BAF312) in Patients With Relapsing-Remitting Multiple Sclerosis. JAMA Neurol. 2016, 73, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Bar-Or, A.; Cree, B.A.C.; Fox, R.J.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnold, D.L.; Arnould, S.; Scherz, T.; et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 2018, 391, 1263–1273. [Google Scholar] [CrossRef]
- Cohen, J.A.; Arnold, D.L.; Comi, G.; Bar-Or, A.; Gujrathi, S.; Hartung, J.P.; Cravets, M.; Olson, A.; Frohna, P.A.; Selmaj, K.W. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 373–381. [Google Scholar] [CrossRef]
- Comi, G.; Kappos, L.; Selmaj, K.W.; Bar-Or, A.; Arnold, D.L.; Steinman, L.; Hartung, H.-P.; Montalban, X.; Havrdová, E.K.; Cree, B.A.C.; et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): A multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019, 18, 1009–1020. [Google Scholar] [CrossRef]
- Yagi, Y.; Nakamura, Y.; Kitahara, K.; Harada, T.; Kato, K.; Ninomiya, T.; Cao, X.; Ohara, H.; Izumi-Nakaseko, H.; Suzuki, K.; et al. Analysis of Onset Mechanisms of a Sphingosine 1-Phosphate Receptor Modulator Fingolimod-Induced Atrioventricular Conduction Block and QT-Interval Prolongation. Toxicol. Appl. Pharmacol. 2014, 281, 39–47. [Google Scholar] [CrossRef]
- Camm, J.; Hla, T.; Bakshi, R.; Brinkmann, V. Cardiac and vascular effects of fingolimod: Mechanistic basis and clinical implications. Am. Hear. J. 2014, 168, 632–644. [Google Scholar] [CrossRef]
- Kastalli, S.; El Aidli, S.; Mourali, S.; Zaiem, A.; Daghfous, R.; Lakhal, M. Cardiac arrhythmia induced by interferon beta-1a. Fundam. Clin. Pharm. 2012, 26, 207–209. [Google Scholar] [CrossRef]
- Hermann, R.; Litwin, J.S.; Friberg, L.E.; Dangond, F.; Munafo, A. Effects of cladribine tablets on heart rate, atrio-ventricular conduction and cardiac repolarization in patients with relapsing multiple sclerosis. Br. J. Clin. Pharm. 2019, 85, 1484–1494. [Google Scholar] [CrossRef]
- Nerrant, E.; Thouvenot, E.; Castelnovo, G. Severe bradycardia: An unreported adverse infusion-associated reaction (IAR) with alemtuzumab. Rev. Neurol. 2017, 173, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Pastuszak, Z.; Tomczykiewicz, K.; Piusinska-Macoch, R.; Stepien, A. Cardiac effects of mitoxanthrone therapy in patients with multiple sclerosis. Kardiol. Pol. 2016, 74, 380–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasheghani-Farahani, A.; Sahraian, M.A.; Darabi, L.; Aghsaie, A.; Minagar, A. Incidence of various cardiac arrhythmias and conduction disturbances due to high dose intravenous methylprednisolone in patients with multiple sclerosis. J. Neurol. Sci. 2011, 309, 75–78. [Google Scholar] [CrossRef]
- Osuagwu, F.; Jahnke, B. Intravenous Methylprednisolone–Induced Nocturnal Sinus Bradycardia in a Multiple Sclerosis Patient. Prim. Care Companion CNS Disord. 2016, 18. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.R.; Gaco, D. Symptomatic Sinus Bradycardia After a Treatment Course Of High-dose Oral Prednisone. J. Emerg. Med. 2013, 45, e55–e58. [Google Scholar] [CrossRef]
- Pavičić, T.; Ruška, B.; Adamec, I.; Habek, M. Recurrent atrial fibrillation after pulse corticosteroid treatment for a relapse of multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 32, 30–32. [Google Scholar] [CrossRef]
- Frahm, N.; Hecker, M.; Zettl, U.K. Polypharmacy in outpatients with relapsing-remitting multiple sclerosis: A single-center study. PLoS ONE 2019, 14, e0211120. [Google Scholar] [CrossRef]
- Piotrowicz, E.; Baranowski, R.; Piotrowska, M.; Zieliński, T.; Piotrowicz, R. Variable Effects of Physical Training of Heart Rate Variability, Heart Rate Recovery, and Heart Rate Turbulence in Chronic Heart Failure. Pacing Clin. Electrophysiol. 2009, 32, S113–S115. [Google Scholar] [CrossRef]
- Ziemssen, T.; Siepmann, T. The Investigation of the Cardiovascular and Sudomotor Autonomic Nervous System-A Review. Front. Neurol. 2019, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Jagannath, V.A.; Pucci, E.; Asokan, G.V.; Robak, E.W. Percutaneous transluminal angioplasty for treatment of chronic cerebrospinal venous insufficiency (CCSVI) in people with multiple sclerosis. Cochrane Database Syst. Rev. 2019, 5, CD009903. [Google Scholar] [CrossRef]
| Reference | Multiple Sclerosis (MS) Patients—n | MS Phenotype | Method | Time Domain Analysis | Frequency Domain Analysis | Results/Conclusion |
|---|---|---|---|---|---|---|
| Shirbani [4] | 23 | Not reported (NR) | Heart rate (HR), HR variability (HRV), blood pressure (BP), baroreflexsensitivity (BRS) at rest | x | x | compared to controls significantly lower baroreceptor sensitivity but higher systolic blood pressure variability in MS |
| Flachenecker [5] | 26 | Relapsing–remitting multiple sclerosis (RRMS) | Heart rate response (HRR), BP response (BPR), Ewing battery | x | parasympathetic dysfunction closely related to progression of disability, but sympathetic dysfunction associated with clinical activity of MS. | |
| Merkelbach [8] | 84 | 54 RRMS, 14 secondary progressive multiple sclerosis (SPMS), 16 primary progressive multiple sclerosis (PPMS) | HRR, BPR, Ewing battery | x | weakly significant correlation between autonomic tests and fatigue scores | |
| Habek [17] | 121 | Clinically isolated syndrome (CIS) | HRR, BPR to Valsalva, deep breathing, tilt table | x | patients with CIS experienced worsening of ANS abnormalities during 2-year follow-up predicted by MRI | |
| Krbot Skoric [18] | 94 | CIS | HRR, BPR to Valsalva, deep breathing, tilt table | x | three predictors for occurrence of new relapse: COMPASS-31 > 7.32, total number of T2 lesions > 3 and decreasing supine level of epinephrine | |
| Hale [27] | 31 | NR | HRR, BPR to Valsalva, deep breathing, tilt table | x | abnormal autonomic function on laboratory testing in 5 MS patients, two of whom with abnormal heart rate response to the cycle test | |
| Kodounis [26] | 33 | NR | HRV, BPR, QTc, Ewing battery | x | 42.42% of the patients demonstrated ANS dysfunction | |
| Videira [28] | 20 | RRMS | HRV, BPR, BRS Ewing battery with tilt test | x | x | HRV showed both parasympathetic and sympathetic impairment in MS |
| Diamond [29] | 18 | NR | HRV at rest | x | x | significantly lower vagal power in MS patients |
| Frontoni [30] | 16 | NR | HRV at rest and tilt-table, HRR, BPR, Ewing battery | x | x | no significant correlation between spectral ANS parameters and lesion area or localization as detected on MRI |
| Linden [31] | 20 | NR | HRR, BPR during deep breathing and tilt table | x | x | spectral and cross-spectral methods are valuable methods for evaluation of cardiovascular regulation |
| Brezinova [32] | 36 | 29 RRMS, 4 SPMS, 3 PPMS | HRV during deep breathing and after active standing | x | x | spectral analysis of HRV in the rest-tilt-rest test differs significantly in MS patients compared to controls |
| Ferini-Strambi [33] | 25 | NR | HRR, BPR, RR interval variation during deep breathing, Valsalva, tilt table, HRV during sleep | x | A reduced parasympathetic activity in MS patients during both rapid eye movement and non-REM sleep | |
| Keller [34] | 11 | RRMS | HR, BP at rest | x | reduced muscle sympathetic nerve activity and plasma norepinephrine in MS patients | |
| Monge-Argiles [35] | 34 | NR | HRV 24-h electrocardiogram (ECG) | x | x | Increased sympathetic tone in MS patients as measured by HRV |
| Giubilei [36] | 20 | RRMS | HRR, BPR Ewing battery, HRV during sleep | x | HRV showed a lower degree of adaptability in MS patients | |
| Tombul [37] | 34 | RRMS | HRV 24-h ECG | x | reduced HRV in MS patients | |
| Ganz [38] | 11 | RRMS | HRR during deep breathing and mental stress | x | Lyapounov exponent | significant lower Lyapounov exponent suggesting less adaptive central autonomic organisation |
| Habek [39] | 104 | CIS | HRV, HRR, BPR to Valsalva, deep breathing, passive tilt | x | x | Autonomic dysfunction in 59.8% of patients, parasympathetic dysfunction in 5%, sympathetic in 42.6% |
| Al Araji [40] | 55 | NR | HRR, BPR, Ewing battery | x | Autonomic symptoms significantly more prevalent in MS patients than in controls | |
| Gunal [41] | 22 | RRMS | HRR, BPR, Ewing battery | x | 90% of the patients had symptoms related to autonomic dysfunction, 45.5% had abnormal cardiovascular autonomic function testing | |
| Thomaides [42] | 10 | SPMS | HRR, BPR during handgrip, Valsalva, deep breathing, tilt and mental stress | x | impaired pressor responses in disabled patients | |
| Saari [43] | 51 | NR | HRR, BPR during normal breathing, Ewing battery with tilt table test | x | decreased HRV during tilt table test and deep breathing in MS patients | |
| Gervasoni [44] | 23 | NR | HRV at rest, standing, light exercise and recovery | x | x | in MS patients HRV slightly but not significantly higher, significantly lower parasympathetic indexes during baseline and post-exercise |
| Adamec [45] | 70 | 40 RRMS, 30 progressive multiple sclerosis (PMS) | HRR and BPR to Valsalva, HRR to deep breathing, BPR to tilt table | x | x | significantly higher total CASS score in PMS patients compared to RRMS |
| Studer [45] | 120 | 84 RRMS, 36 PMS | HRV during rest and tilt table test | x | x | sympathetic dysfunction as measured by HRV closely related to the progression of disability in MS patients |
| de Seze [46] | 75 | 25 RRMS, 25 SPMS, 25 PPMS | HRV, BPR during deep breathing, Valsalva, standing up test | x | correlation of autonomic dysfunction with spinal cord atrophy | |
| Vita [47] | 40 | NR | HRV, BPR, R-R interval variation at rest and Ewing battery | x | significant association between presence of autonomic dysfunction and clinical and MRI evidence of brainstem lesions | |
| Brinar [48] | 28 | RRMS | HRR during Valsalva and cortical activation, HRR and BPR during quick standing, | x | positive correlation between autonomic dysfunction and MRI findings | |
| Mahovic [49] | 39 | RRMS | HRV 24-h ECG | x | x | significantly lower HRV in MS patients than in controls |
| Damla [50] | 51 | RRMS | HRV 24-h ECG | x | x | decreased HRV in MS compared to controls, but no correlation with disability or disease activity |
| Vlcek [51] | 19 | RRMS | HRV during Stroop test | x | x | lower HR and lower epinephrine increment after Stroop test in MS patients compared to controls |
| Nasseri [52] | 46 | 20 RRMS, 26 SPMS | HRR during deep breathing, standing up test and Valsalva | x | progression of autonomic dysfunction over 1 year | |
| Nasseri [53] | 20 | RRMS | HRR during deep breathing, standing up test and Valsalva | x | progression of autonomic dysfunction over 2 years without significant progression of EDSS | |
| Krbot Skoric [54] | 70 | NR | HRR and BPR to Valsalva and Tilt table, HRV to deep breathing | x | clear association between fatigue and scores in subjective tests but only modest correlation between fatigue and objective test of the ANS | |
| Lebre [55] | 50 | RRMS | HRV, BPR, Ewing battery | x | reduced capacity to increase blood pressure in MS patients with fatigue | |
| Flachenecker [56] | 60 | NR | HRV, BPR, Ewing battery | x | x | autonomic responses correlated with fatigue |
| Keselbrenner [57] | 10 | NR | HRV, BPR at rest and standing | x | x | age-related reduction in vagal activity occurred earlier in patients with MS who experienced fatigue |
| Heesen [58] | 23 | 19 RRMS, 3 SPMS, 1 RPMS | HRV during stress | x | cognitive stress induces IFNg production in healthy controls but not in MS patients with fatigue, reduced cardiac response in MS patients | |
| Sander [59] | 53 | 36 RRMS, 10 SPMS, 7 PPMS | HRV during vigilance task | x | x | Cognitive fatigue in MS is related to parasympathetic activity |
| Niepel [60] | 26 | 21 RRMS, 3 SPMS, 2 PPMS | HR and BP in sitting position and during handgrip before and after modafinil | x | MS patients with fatigue had a reduced level of cardiovascular sympathetic activation | |
| Arata [61] | 21 | 10 RRMS, 5 SPMS, 6 PPMS | HRV during deep breathing, Valsalva and after active standing | x | improvement of autonomic dysfunction after balloon angioplasty of cerebral veins | |
| Huang [62] | 10 | RRMS | BPR and HRR to carotid baroreflex stimulation | x | impaired baroreflex control of blood pressure in MS patients | |
| Sanya [63] | 13 | RRMS | BPR and HRR to carotid baroreflex stimulation | x | x | baroreflex dysfunction in MS patients |
| Winder [64] | 74 | RRMS | HRV, BPR at rest | x | x | association between increased sympathetic modulation and left insular and hippocampal lesions |
| Simula [65] | 27 | RRMS | HRV 24-h ECG | x | x | fingolimod dosing shifts cardiac autonomic regulation towards sympathetic predominance |
| Vehoff [66] | 33 | RRMS | HRV, BPR during breathing at rest, deep breathing, Valsalva before and after fingolimod | x | initial increase in HRV, measured 4.5 h after first intake of fingolimod, but substantial decrease in HRV within next 3 months | |
| Akbulak [67] | 64 | RRMS | HRV 24-h ECG | x | x | decreased HRV up to 72 h after fingolimod intake |
| Rossi [68] | 55 | RRMS | HRV, BPR Ewing battery with tilt table | x | x | significant correlations between measures of parasympathetic function and fingolimod-induced bradycardia |
| Li [69] | 78 | RRMS | HRV at rest before and after Fingolimod | x | x | increase of HRV parameters representing parasympathetic activities two hours after fingolimod administration |
| Vanoli [70] | 625 | RRMS | HRV at rest before and after Fingolimod | x | x | normalized spectral power in the high-frequency band and previous annualized relapse rate were independently correlated with the probability of undergoing extended monitoring |
| Hilz [71] | 21 | RRMS | HRV, BPR, Ewing battery | x | x | patients with higher resting BP and higher BP increase during handgrip-exercise had prolonged HR slowing after Fingolimod |
| Tran [72] | 113 | RRMS | HRV 24-h ECG | x | x | ozanimod does not prolong QTc interval |
| Racca [73] | 10 | RRMS | HRV, BP after baroreflex stimulation | x | x | no modification of baroreflex sensitivity after first dose of Fingolimod |
| Hilz [74] | 21 | RRMS | HRV, BP at rest before and after fingolimod | x | x | increases in parasympathetic and cardiac autonomic modulation after fingolimod |
| FREEDOMS | TRANSFORMS | |||||
|---|---|---|---|---|---|---|
| Fingolimod | Placebo (n = 418) | Fingolimod | Placebo (n = 431) | |||
| 1.25 mg (n= 429) | 0.5 mg (n = 425) | 1.25 mg (n= 420) | 0.5 mg (n = 429) | |||
| Cardiovascular adverse events—n (%) | ||||||
| Hypertension | 27 (6.3) | 26 (6.1) | 16 (3.8) | 21 (5.0) | 16 (3.7) | 8 (1.9) |
| Bradycardia, bradyarrhythmia, or sinus bradycardia | 14 (3.3) | 9 (2.1) | 3 (0.7) | 10 (2.4) | 2 (0.5) | 0 |
| First degree atrioventricular (AV) block | 5 (1.2) | 2 (0.5) | 2 (0.5) | 3 (0.7) | 1 (0.2) | 0 |
| Second degree AV block | 1 (0.2) | 0 | 1 (0.2) | 2 (0.5) | 1 (0.2) | 0 |
| Cardiac monitoring | ||||||
| Maximum first-dose heart rate decrease—bpm | 10 | 8 | – | 12 | 8 | – |
| First degree AV block—n (%) | 37 (8.6) | 20 (4.7) | 6 (1.4) | – | – | – |
| Second degree AV block—n (%) | 4 (0.9) | 1 (0.2) | 0 | – | – | – |
| Mean blood pressure increase in first 6 months | 3.6/2.1 mmHg | 1.9/0.7 mmHg | – | 3 mmHg | 2 mmHg | – |
| Fingolimod | Placebo (n = 355) | ||
|---|---|---|---|
| 1.25 mg (n = 370) | 0.5 mg (n = 358) | ||
| First dose monitoring events | |||
| Bradycardia | 21 (6%) | 5 (1%) | 1 (<0.5%) |
| Symptomatic bradycardia | 15 (4%) | 3 (1%) | 1 (<0.5%) |
| First-degree atrioventricular (AV) block | 35 (10%) | 17 (5%) | 7 (2%) |
| Mobitz I AV block | 6 (2%) | 0 | 0 |
| Second-degree AV block | 1 (<0.5%) | 0 | 0 |
| 24-h Holter monitoring events | |||
| First dose | |||
| Mobitz I (Wenckebach) second-degree AV block | 24 (7%) | 13 (4%) | 7 (2%) |
| Second-degree AV block | 12 (3%) | 7 (2%) | 0 |
| 3 months | |||
| Mobitz I (Wenckebach) second-degree AV block | 0 | 0 | 5 (2%) |
| Second-degree AV block | 0 | 0 | 1 (<0.5%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Findling, O.; Hauer, L.; Pezawas, T.; Rommer, P.S.; Struhal, W.; Sellner, J. Cardiac Autonomic Dysfunction in Multiple Sclerosis: A Systematic Review of Current Knowledge and Impact of Immunotherapies. J. Clin. Med. 2020, 9, 335. https://doi.org/10.3390/jcm9020335
Findling O, Hauer L, Pezawas T, Rommer PS, Struhal W, Sellner J. Cardiac Autonomic Dysfunction in Multiple Sclerosis: A Systematic Review of Current Knowledge and Impact of Immunotherapies. Journal of Clinical Medicine. 2020; 9(2):335. https://doi.org/10.3390/jcm9020335
Chicago/Turabian StyleFindling, Oliver, Larissa Hauer, Thomas Pezawas, Paulus S. Rommer, Walter Struhal, and Johann Sellner. 2020. "Cardiac Autonomic Dysfunction in Multiple Sclerosis: A Systematic Review of Current Knowledge and Impact of Immunotherapies" Journal of Clinical Medicine 9, no. 2: 335. https://doi.org/10.3390/jcm9020335





